Quality management system: A tool for the development of the organization or an additional burden?
- Autores: Zayunchkovskiy S.Y.1, Konovalov S.A.1, Zinchenko V.V.1, Sharova D.E.1, Ahkmad E.S.1, Vladzymyrskyy A.V.1
-
Afiliações:
- Moscow Center for Diagnostics and Telemedicine
- Edição: Volume 4, Nº 3 (2023)
- Páginas: 439-447
- Seção: Correspondence
- URL: https://journals.rcsi.science/DD/article/view/254081
- DOI: https://doi.org/10.17816/DD514629
- ID: 254081
Citar
Resumo
A quality management system constitutes one of the organization’s management systems that provides for the selection of a set of processes in the organization’s activities designed to ensure the stable quality of products and services provided.
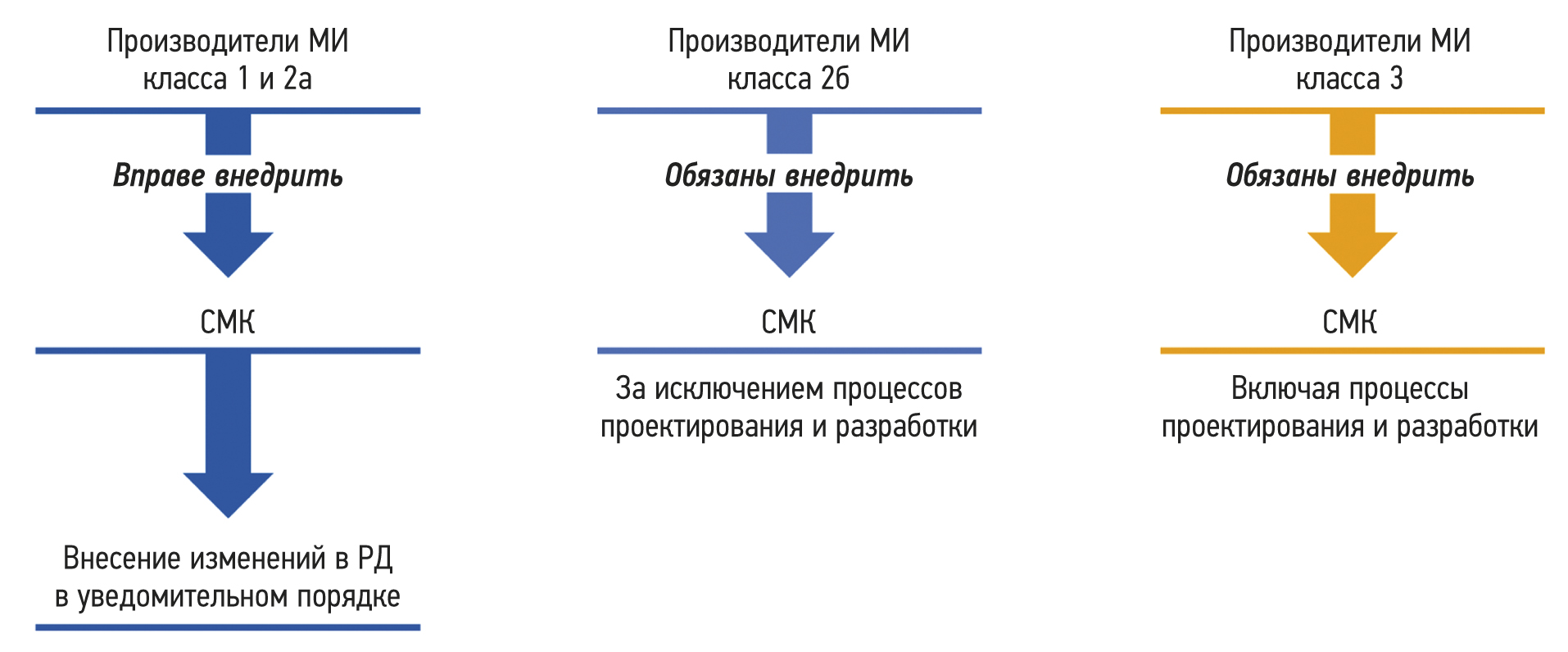
The growth of global industrial production has underscored the need for the creation of such production and management systems. These systems are designed to ensure that enterprises remains prepared to meet the constantly changing consumer value of manufactured products in accordance with consumer requirements, as well as the satisfaction of consumers themselves. As a result, attention began to focus on the production processes implemented within the organization when creating products. Regarding the production of medical devices, a quality management system can be defined as an organizational structure encompassing its functions, procedures, processes, and resources necessary for the coordinated direction and management of a manufacturing organization with respect to the quality of medical products.
The article reflects the principles of the quality management system and management processes. Noteworthy emphasis is placed on the features of quality management systems for medical devices, including the features of the quality management system for software that is a medical device. Furthermore, the conditions under which the quality management system becomes a tool for ensuring the sustainable development of the organization are noted.
Palavras-chave
Texto integral
##article.viewOnOriginalSite##Sobre autores
Sergey Zayunchkovskiy
Moscow Center for Diagnostics and Telemedicine
Email: ZayunchkovskijSY@zdrav.mos.ru
ORCID ID: 0009-0002-7463-7699
Rússia, Moscow
Sergey Konovalov
Moscow Center for Diagnostics and Telemedicine
Email: KonovalovSA4@zdrav.mos.ru
ORCID ID: 0009-0003-0011-3371
Rússia, Moscow
Viktoria Zinchenko
Moscow Center for Diagnostics and Telemedicine
Autor responsável pela correspondência
Email: ZinchenkoVV1@zdrav.mos.ru
ORCID ID: 0000-0002-2307-725X
Código SPIN: 4188-0635
Rússia, Moscow
Daria Sharova
Moscow Center for Diagnostics and Telemedicine
Email: SharovaDE@zdrav.mos.ru
ORCID ID: 0000-0001-5792-3912
Código SPIN: 1811-7595
Rússia, Moscow
Ekaterina Ahkmad
Moscow Center for Diagnostics and Telemedicine
Email: AkhmadES@zdrav.mos.ru
ORCID ID: 0000-0002-8235-9361
Código SPIN: 5891-4384
Rússia, Moscow
Anton Vladzymyrskyy
Moscow Center for Diagnostics and Telemedicine
Email: VladzimirskijAV@zdrav.mos.ru
ORCID ID: 0000-0002-2990-7736
Código SPIN: 3602-7120
MD, Dr. Sci. (Med.)
Rússia, MoscowBibliografia
- Mikhailov YuI. Process management in the quality management system of the enterprise. Discourse. 2017;(6):51–57. (In Russ).
- Zinovieva EV, Sapunova AV, Ivanov IV. Safety of circulation of medical devices at all stages of their life cycle. Public health. 2022;2(3):16–24. (In Russ). doi: l0.21045/2782-1676-202l-2-3-16-24
- Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC // Official J Eur Union. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0745. Accessed: 15.07.2023.
- Lim K, Heo TY, Yun J. Trends in the approval and quality management of artificial intelligence medical devices in the Republic of Korea. Diagnostics (Basel). 2022;12(2):355. doi: 10.3390/diagnostics12020355
- Gusev AV, Vladzimirsky AV, Sharova DE, et al. Development of research and development in the field of artificial intelligence technologies for healthcare in the Russian Federation: Results of 2021. Digital Diagnostics. 2022;3(3):178–194. (In Russ). doi: 10.17816/DD107367
Arquivos suplementares