Modeling of mixed infection with Zika and West Nile viruses (Flaviviridae: Orthoflavivirus: Orthoflavivirus zikaense, Orthoflavivirus nilense) in vitro and in vivo
- Authors: Svyatchenko V.A.1, Protopopova E.V.1, Legostaev S.S.1, Mikryukova T.P.1, Agafonov A.P.1, Loktev V.B.1
-
Affiliations:
- State Research Center of Virology and Biotechnology «Vector»
- Issue: Vol 70, No 4 (2025)
- Pages: 340-348
- Section: ORIGINAL RESEARCHES
- URL: https://journals.rcsi.science/0507-4088/article/view/330071
- DOI: https://doi.org/10.36233/0507-4088-324
- EDN: https://elibrary.ru/gkebwv
- ID: 330071
Cite item
Abstract
Introduction. Mosquito-borne human diseases caused by Zika virus and West Nile virus (WNV) are widespread across multiple continents and cause major outbreaks. Their ranges overlap and the possibility of mixed infections is obvious. The information of such mixed infections is limited.
The aim of the study is to investigate the features of mixed infection of WNV and Zika virus in vitro and in vivo in order to assess their possible interference and/or enhancement of viral infection.
Materials and methods. The study used West Nile virus and Zika virus strains Vlg27924 and MR766, respectively. The infectious activity of viruses during mono- and co-infection was determined on Vero E6 cell culture using RT-PCR, as well as on BALB/c mice using various administration schemes.
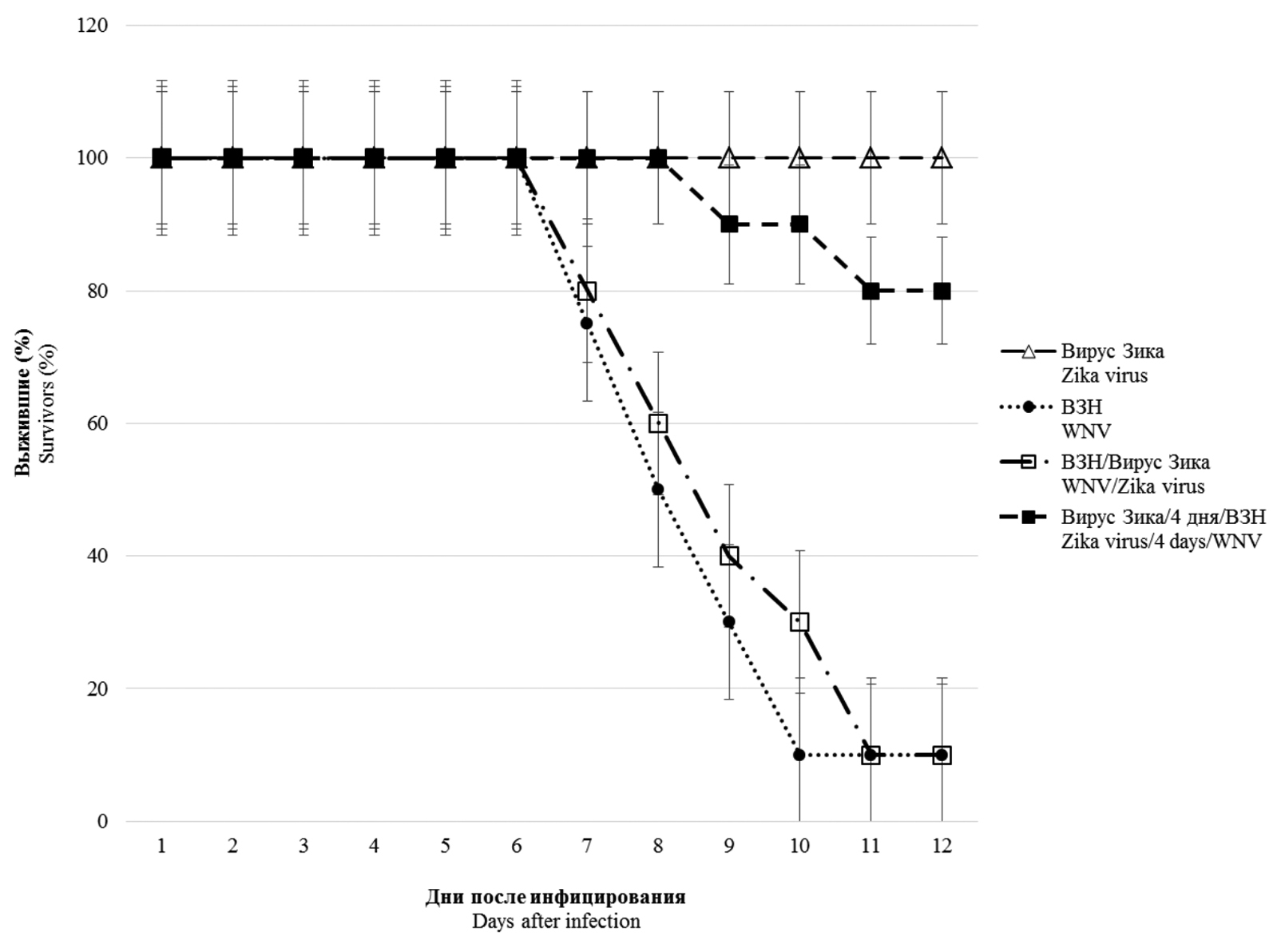
Results. In vitro studies of co-infection with WNV and Zika virus showed that co-infection leads to interference, with the degree of competitive inhibition of replication being more pronounced for Zika virus, reaching 1000 times or more when compared to mono-infection. During simultaneous infection in mice, Zika virus does not affect the development of lethal infection caused by WNV. However, preliminary (4 and 20 days) infection with a sublethal dose of Zika virus reliably protects animals from subsequent administration of 10 and 100 LD50 WNV, respectively. In pre-infected and co-infected animals with Zika virus, the development of WNV-specific viral neutralizing antibodies was recorded in higher titers than in WNV monoinfected animals.
Conclusion. The presence of in vitro interference between the studied orthoflaviviruses was shown, most pronounced in relation to the Zika virus. No significant effect was observed with simultaneous co-infection in vivo. However, pre-infection of mice with Zika virus provides protection to animals from lethal WNV infection due to the induction of high levels of antibodies that specifically neutralize its infectious activity.
Keywords
Full Text
##article.viewOnOriginalSite##About the authors
Victor A. Svyatchenko
State Research Center of Virology and Biotechnology «Vector»
Email: svyat@vector.nsc.ru
ORCID iD: 0000-0002-2729-0592
Candidate of Biological Sciences, Leading Researcher, Department of Molecular Virology of Flaviviruses and Viral Hepatitis
Russian Federation, 630559, Koltsovo, Novosibirsk RegionElena V. Protopopova
State Research Center of Virology and Biotechnology «Vector»
Email: protopopova_ev@vector.nsc.ru
ORCID iD: 0000-0002-2782-8364
Candidate of Biological Sciences, Leading Researcher, Department of Molecular Virology of Flaviviruses and Viral Hepatitis
Russian Federation, 630559, Koltsovo, Novosibirsk RegionStanislav S. Legostaev
State Research Center of Virology and Biotechnology «Vector»
Email: legostaev_ss@vector.nsc.ru
ORCID iD: 0000-0002-6202-445X
junior researcher of the department of molecular virology of flaviviruses and viral hepatitis
Russian Federation, 630559, Koltsovo, Novosibirsk RegionTamara P. Mikryukova
State Research Center of Virology and Biotechnology «Vector»
Email: mikryukova_tp@vector.nsc.ru
ORCID iD: 0000-0003-4350-4260
Candidate of Biological Sciences, Senior Researcher, Department of Molecular Virology of Flaviviruses and Viral Hepatitis
Russian Federation, 630559, Koltsovo, Novosibirsk RegionAlexander P. Agafonov
State Research Center of Virology and Biotechnology «Vector»
Email: agafonov@vector.nsc.ru
ORCID iD: 0000-0003-2577-0434
Doctor of Biological Sciences, Director General
Russian Federation, 630559, Koltsovo, Novosibirsk RegionValery B. Loktev
State Research Center of Virology and Biotechnology «Vector»
Author for correspondence.
Email: loktev@vector.nsc.ru
ORCID iD: 0000-0002-0229-321X
MD, PhD, DSc, Prof., academician RANS, Chief Researcher, Department of Molecular Virology of Flaviviruses and Viral Hepatitis
Russian Federation, 630559, Koltsovo, Novosibirsk RegionReferences
- Postler T.S., Beer M., Blitvich B.J., Bukh J., de Lamballerie X., Drexler J.F., et al. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch. Virol. 2023; 168(9): 224. https://doi.org/10.1007/s00705-023-05835-1
- Baker R.E., Mahmud A.S., Miller I.F., Rajeev M., Rasambainarivo F., Rice B.L., et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022; 20(4): 193–05. https://doi.org/10.1038/s41579-021-00639-z
- Petersen L.R., Brault A.C., Nasci R.S. West Nile virus: review of the literature. JAMA. 2013; 310(3): 308–15. https://doi.org/10.1001/jama.2013.8042
- van Leur S.W., Heunis T., Munnur D., Sanyal S. Pathogenesis and virulence of flavivirus infections. Virulence. 2021; 12(1): 2814–38. https://doi.org/10.1080/21505594.2021.1996059
- Brüssow H., Figuerola J. The spread of the mosquito-transmitted West Nile virus in North America and Europe. Microb. Biotechnol. 2025; 18(3): e70120. https://doi.org/10.1111/1751-7915.70120
- Kariwa H., Murata R., Totani M., Yoshii K., Takashima I. Increased pathogenicity of West Nile virus (WNV) by glycosylation of envelope protein and seroprevalence of WNV in wild birds in Far Eastern Russia. Int. J. Environ. Res. Public Health. 2013; 10(12): 7144–64. https://doi.org/10.3390/ijerph10127144
- Korobitsyn I.G., Moskvitina N.S., Tyutenkov O.Y., Gashkov S.I., Kononova Y.V., Moskvitin S.S., et al. Detection of tick-borne pathogens in wild birds and their ticks in Western Siberia and high level of their mismatch. Folia Parasitol. (Praha). 2021; 68: 2021.024. https://doi.org/10.14411/fp.2021.024
- Duggal N.K., Langwig K.E., Ebel G.D., Brault A.C. On the fly: interactions between birds, mosquitoes, and environment that have molded West Nile virus genomic structure over two decades. J. Med. Entomol. 2019; 56(6): 1467–74. https://doi.org/10.1093/jme/tjz112
- Dick G.W.A., Kitchen S.F., Haddow A.J., Zika virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952; 46: 509–20. https://doi.org/10.1016/0035-9203(52)90042-4
- Rabe I.B., Hills S.L., Haussig J.M., Walker A.T., Dos Santos T., San Martin J.L., et al. A review of the recent epidemiology of Zika virus infection. Am. J. Trop. Med. Hyg. 2025; 112(5): 1026–35. https://doi.org/10.4269/ajtmh.24-0420
- Pielnaa P., Al-Saadawe M., Saro A., Dama M.F., Zhou M., Huang Y., et al. Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology. 2020; 543: 34–42. https://doi.org/10.1016/j.virol.2020.01.015
- Rückert C., Weger-Lucarelli J., Garcia-Luna S.M., Young M.C., Byas A.D., Murrieta R.A., et al. Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat. Commun. 2017; 8: 15412. https://doi.org/10.1038/ncomms15412
- Laredo-Tiscareño S.V., Machain-Williams C., Rodríguez-Pérez M.A., Garza-Hernandez J.A., Doria-Cobos G.L., Cetina-Trejo R.C., et al. Arbovirus surveillance near the Mexico–us border: Isolation and sequence analysis of chikungunya virus from patients with dengue-like symptoms in Reynosa, Tamaulipas. Am. J. Trop. Med. Hyg. 2018; 99(1): 191–94. https://doi.org/10.4269/ajtmh.18-0117
- Cherabuddi K., Iovine N.M., Shah K., White S.K., Paisie T., Salemi M., et al. Zika and Chikungunya virus co-infection in a traveller returning from Colombia, 2016: virus isolation and genetic analysis. JMM Case Rep. 2016; 3(6): e005072. https://doi.org/10.1099/jmmcr.0.005072
- Iovine N.M., Lednicky J., Cherabuddi K., Crooke H., White S.K., Loeb J.C., et al. Coinfection with Zika and Dengue-2 viruses in a traveler returning from Haiti, 2016: clinical presentation and genetic analysis. Clin. Infect. Dis. 2017; 64(1): 72–5. https://doi.org/10.1093/cid/ciw667
- Laredo-Tiscareño S.V., Garza-Hernandez J.A., Salazar M.I., De Luna-Santillana E.J., Tangudu C.S., Cetina-Trejo R.C., et al. Surveillance for flaviviruses near the Mexico-U.S. border: co-circulation of Dengue virus serotypes 1, 2, and 3 and West Nile virus in Tamaulipas, Northern Mexico, 2014–2016. Am. J. Trop. Med. Hyg. 2018; 99(5): 1308–17. https://doi.org/10.4269/ajtmh.18-0426
- Slavov S.N., Gonzaga F.A.C., Pimentel B.M.S., Ramos D.D.A.R., de Araújo W.N., Covas D.T., et al. Zika virus RNA surveillance in blood donors in the Federal District of Brazil during the 2016 outbreak. Hematol. Transfus. Cell Ther. 2020; 42(4): 394–96. https://doi.org/10.1016/j.htct.2019.08.006
- Vasilakis N., Shell E.J., Fokam E.B., Mason P.W., Hanley K.A., Estes D.M., et al. Potential of ancestral sylvatic dengue-2 viruses to re-emerge. Virology. 2007; 358(2): 402–12. https://doi.org/10.1016/j.virol.2006.08.049
- Aaskov J., Buzacott K., Thu H.M., Lowry K., Holmes E.C. Long-term transmission of defective RNA viruses in humans and Aedes mosquitoes. Science. 2006; 311(5758): 236–38. https://doi.org/10.1126/science.1115030
- Paz-Bailey G., Adams L.E., Deen J., Anderson K.B., Katzelnick L.C. Dengue. Lancet. 2024; 403(10427): 667–82. https://doi.org/10.1016/S0140-6736(23)02576-X
- Rodriguez-Morales A.J., Villamil-Gómez W.E., Franco-Paredes C. The arboviral burden of disease caused by co-circulation and co-infection of dengue, chikungunya and Zika in the Americas. Travel Med. Infect. Dis. 2016; 14(3): 177–9. https://doi.org/10.1016/j.tmaid.2016.05.004
- Svyatchenko V.A., Nikonov S.D., Mayorov A.P., Gelfond M.L., Loktev V.B. Antiviral photodynamic therapy: inactivation and inhibition of SARS-CoV-2 in vitro using methylene blue and Radachlorin. Photodiagnosis Photodyn. Ther. 2021; 33: 102112. https://doi.org/10.1016/j.pdpdt.2020.102112
- Toth K., Spencer J.F., Dhar D., Sagartz J.E., Buller R.M., Painter G.R., et al. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus induced mortality in a permissive, immunosuppressed animal model. Proc. Natl. Acad. Sci. USA. 2008; 105(20): 7293–97. https://doi.org/10.1073/pnas.0800200105
- Svyatchenko V.A., Ternovoi V.A., Lutkovskiy R.Y., Protopopova E.V., Gudymo A.S., Danilchenko N.V., et al. Human adenovirus and influenza A virus exacerbate SARS-CoV-2 infection in animal models. Microorganisms. 2023; 11(1): 180. https://doi.org/10.3390/microorganisms11010180
- Sanders R., Mason D.J., Foy C.A., Huggett J.F. Evaluation of digital PCR for absolute RNA quantification. PLoS One. 2013; 8(9): e75296. https://doi.org/10.1371/journal.pone.0075296
- Petrović T., Blazquez A.B., Lupulović D., Lazić G., Escribano-Romero E., Fabijan D., et al. Monitoring West Nile virus (WNV) infection in wild birds in Serbia during 2012: first isolation and characterisation of WNV strains from Serbia. Euro. Surveill. 2013; 18(44): 20622. https://doi.org/10.2807/1560-7917.es2013.18.44.20622
- DaPalma T., Doonan B.P., Trager N.M., Kasman L.M. A systematic approach to virus-virus interactions. Virus Res. 2010; 149(1): 1–9. https://doi.org/10.1016/j.virusres.2010.01.002
- Zhu Y., He Z., Qi Z. Virus-host Interactions in early Japanese encephalitis virus infection. Virus Res. 2023; 331: 199120. https://doi.org/10.1016/j.virusres.2023.199120
- Du Y., Wang C., Zhang Y. Viral coinfections. Viruses. 2022; 14(12): 2645. https://doi.org/10.3390/v14122645
- Gallichotte E.N., Fitzmeyer E.A., Williams L., Spangler M.C., Bosco-Lauth A.M., Ebel G.D. WNV and SLEV coinfection in avian and mosquito hosts: impact on viremia, antibody responses, and vector competence. J. Virol. 2025; 98(10): e0104124. https://doi.org/10.1128/jvi.01041-24
- Dejnirattisai W., Supasa P., Wongwiwat W., Rouvinski A., Barba-Spaeth G., Duangchinda T., et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016; 17(9): 1102–08. https://doi.org/10.1038/ni.3515
- Stettler K., Beltramello M., Espinosa D.A., Graham V., Cassotta A., Bianchi S., et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016; 353(6301): 823–26. https://doi.org/10.1126/science.aaf8505
- Lobigs M., Diamond M.S. Feasibility of cross-protective vaccination against flaviviruses of the Japanese encephalitis serocomplex. Expert Rev. Vaccines. 2012; 11(2): 177–87. https://doi.org/10.1586/erv.11.180
- Sapparapu G., Fernandez E., Kose N., Bin C., Fox J.M., Bombardi R.G., et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 2016; 540(7633): 443–7. https://doi.org/10.1038/nature20564
- Garg H., Yeh R., Watts D.M., Mehmetoglu-Gurbuz T., Resendes R., Parsons B., et al. Enhancement of Zika virus infection by antibodies from West Nile virus seropositive individuals with no history of clinical infection. BMC Immunol. 2021; 22(1): 5. https://doi.org/10.1186/s12865-020-00389-2
Supplementary files