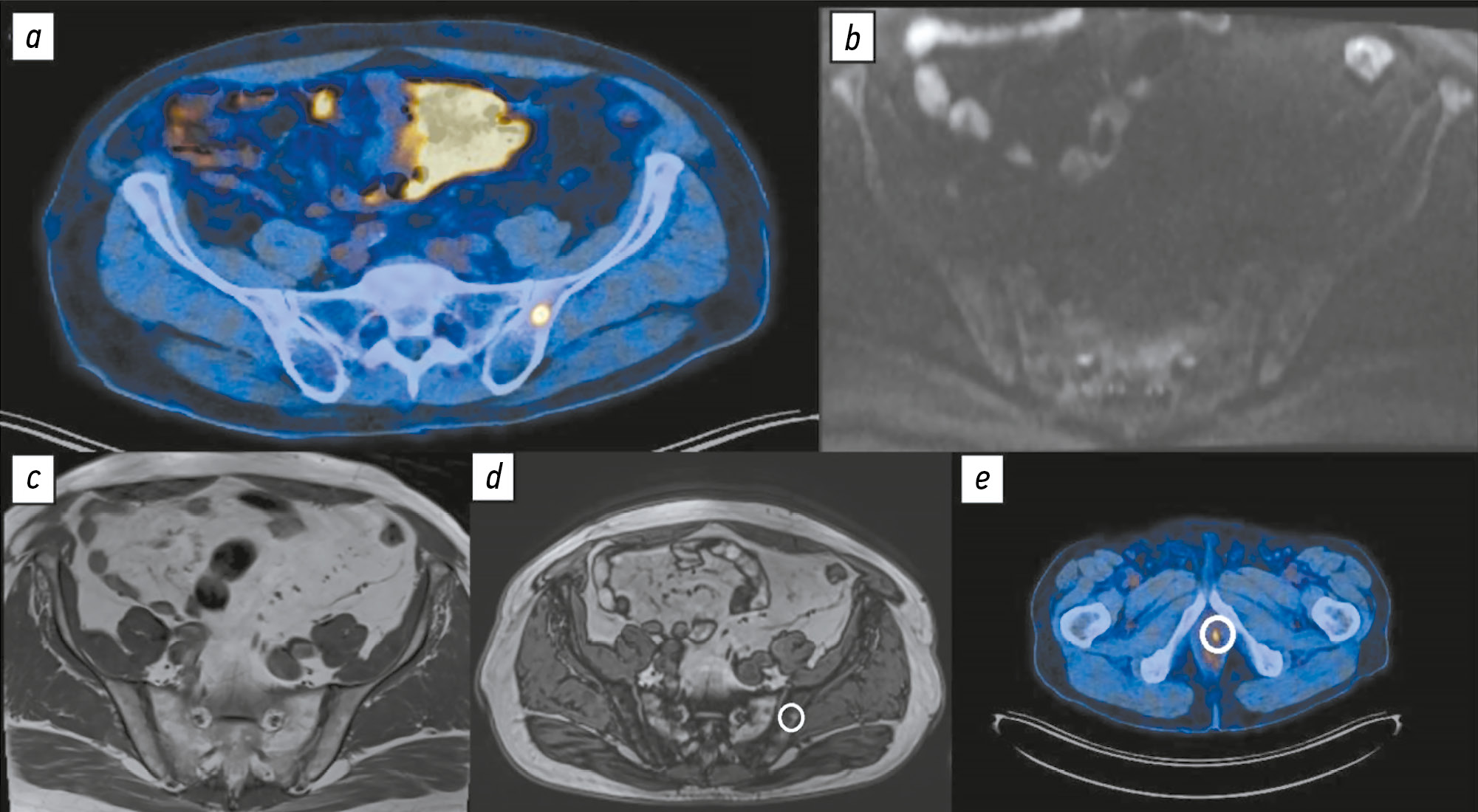
Comparison of the diagnostic accuracy of whole-body diffusion-weighted imaging and 18F-prostate-specific membrane antigen-1007 positron emission tomography combined with computed tomography for detecting bone metastases in prostate cancer
- Authors: Gelezhe P.B.1,2, Reshetnikov R.V.1, Blokhin I.A.1, Kodenko M.R.1
-
Affiliations:
- Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
- European Medical Center
- Issue: Vol 6, No 2 (2025)
- Pages: 239-250
- Section: Original Study Articles
- URL: https://journals.rcsi.science/DD/article/view/310213
- DOI: https://doi.org/10.17816/DD633391
- EDN: https://elibrary.ru/QXLAWR
- ID: 310213
Cite item
Full Text
Abstract
BACKGROUND: The increasing availability of 18F-prostate-specific membrane antigen-1007 (18F-PSMA-1007) for prostate cancer staging highlighted its advantages, particularly its higher spatial resolution compared to analogs. Moreover, accumulating scientific data indicate an increase in false-positive findings, predominantly in bones, which may lead to unwarranted upstaging of the disease. Diffusion-weighted imaging may be used for the early detection of bone metastases.
AIM: This study aimed to assess and compare the diagnostic accuracy of whole-body 18F-PSMA-1007 positron emission tomography combined with computed tomography and whole-body and pelvic bone diffusion-weighted imaging in patients with prostate cancer.
METHODS: A retrospective single-center selective study was conducted. The imaging results of 119 patients with prostate cancer were divided into two groups: group 1 comprised 40 pairs of 18F-PSMA-1007 positron emission tomography combined with computed tomography and whole-body diffusion-weighted magnetic resonance imaging scans, and group 2 included 79 pairs of similar studies, with magnetic resonance imaging performed only for the pelvic bones. The diagnostic studies were performed at an inter-study interval ≤14 days. The metastatic bone lesions detected in different anatomical regions was counted using data from 18F-PSMA-1007 positron emission tomography combined with computed tomography and magnetic resonance imaging. Lesions were considered true positives if confirmed by additional magnetic resonance imaging pulse sequences and/or follow-up observation.
RESULTS: Whole-body diffusion-weighted imaging demonstrated higher specificity (58.1%) for detecting bone metastases than 18F-PSMA-1007 positron emission tomography combined with computed tomography (51.06%). However, its sensitivity was lower: 93.22% versus 97.55%.
CONCLUSION: Despite its advantages, 18F-PSMA-1007 positron emission tomography combined with computed tomography shows a high rate of false-positive bone findings. These are most commonly noted in the ribs, vertebrae, and pelvic bones. Suspicious bone lesions should be further evaluated to avoid unjustified disease upstaging. Thus, whole-body magnetic resonance imaging with diffusion-weighted sequences and selective fat signal suppression can be used.
Full Text
##article.viewOnOriginalSite##About the authors
Pavel B. Gelezhe
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies; European Medical Center
Author for correspondence.
Email: gelezhe.pavel@gmail.com
ORCID iD: 0000-0003-1072-2202
SPIN-code: 4841-3234
MD, Cand. Sci. (Medicine)
Russian Federation, Moscow; MoscowRoman V. Reshetnikov
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Email: ReshetnikovRV1@zdrav.mos.ru
ORCID iD: 0000-0002-9661-0254
SPIN-code: 8592-0558
Cand. Sci. (Physics and Mathematics)
Russian Federation, MoscowIvan A. Blokhin
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Email: BlokhinIA@zdrav.mos.ru
ORCID iD: 0000-0002-2681-9378
SPIN-code: 3306-1387
Russian Federation, Moscow
Maria R. Kodenko
Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies
Email: KodenkoM@zdrav.mos.ru
ORCID iD: 0000-0002-0166-3768
SPIN-code: 5789-0319
Cand. Sci. (Engineering)
Russian Federation, MoscowReferences
- Petersen LJ, Zacho HD. PSMA PET for primary lymph node staging of intermediate and high-risk prostate cancer: an expedited systematic review. Cancer Imaging. 2020;20(1):1–8. doi: 10.1186/s40644-020-0290-9 EDN: EWACNH
- Wondergem M, van der Zant FM, Broos WAM, Knol RJJ. Clinical impact of PSMA PET in biochemically recurrent prostate cancer; a review of the literature. Tijdschrift voor Urologie. 2020;10(6-7):109–121. doi: 10.1007/s13629-020-00296-6 EDN: XRLHSC
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. The Lancet. 2020;395(10231):1208–1216. doi: 10.1016/s0140-6736(20)30314-7 EDN: IDQIFB
- Treglia G, Annunziata S, Pizzuto DA, et al. Detection rate of 18F-Labeled PSMA PET/CT in biochemical recurrent prostate cancer: a systematic review and a meta-analysis. Cancers. 2019;11(5):710. doi: 10.3390/cancers11050710
- Donswijk ML, van Leeuwen PJ, Vegt E, et al. Clinical impact of PSMA PET/CT in primary prostate cancer compared to conventional nodal and distant staging: a retrospective single center study. BMC Cancer. 2020;20(1):1–10. doi: 10.1186/s12885-020-07192-7 EDN: QXMNJG
- The FDA approves PSMAtargeted drug for PET imaging in men with prostate cancer. BJU International. 2021;127(3):267–268. doi: 10.1111/bju.15361
- Caribé PRRV, Koole M, D'Asseler Y, et al. NEMA NU 2-2007 performance characteristics of GE Signa integrated PET/MR for different PET isotopes. EJNMMI Physics. 2019;1(6):11. doi: 10.1186/s40658-019-0247-x
- Giesel FL, Hadaschik B, Cardinale J, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. European Journal of Nuclear Medicine and Molecular Imaging. 2016;44(4):678–688. doi: 10.1007/s00259-016-3573-4 EDN: RQYCMY
- Kroenke M, Mirzoyan L, Horn T, et al. Matched-pair comparison of 68Ga-PSMA-11 and 18F-rhPSMA-7 PET/CT in patients with primary and biochemical recurrence of prostate cancer: frequency of non–tumor-related uptake and tumor positivity. Journal of Nuclear Medicine. 2020;62(8):1082–1088. doi: 10.2967/jnumed.120.251447 EDN: EQTOAN
- Kwee TC, Takahara T, Ochiai R, et al. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. European Radiology. 2008;18(9):1937–1952. doi: 10.1007/s00330-008-0968-z EDN: BJSYMC
- Parker C, Tunariu N, Tovey H, et al. Radium-223 in metastatic castration-resistant prostate cancer: whole-body diffusion-weighted magnetic resonance imaging scanning to assess response. JNCI Cancer Spectrum. 2023;7(6):pkad077. doi: 10.1093/jncics/pkad077 EDN: AZWTFB
- Dresen RC, De Vuysere S, De Keyzer F, et al. Whole-body diffusion-weighted MRI for operability assessment in patients with colorectal cancer and peritoneal metastases. Cancer Imaging. 2019;19(1):1–10. doi: 10.1186/s40644-018-0187-z EDN: IEYSWA
- Yamamoto S, Yoshida S, Ishii C, et al. Metastatic diffusion volume based on apparent diffusion coefficient as a prognostic factor in castration-resistant prostate cancer. Journal of Magnetic Resonance Imaging. 2021;54(2):401–408. doi: 10.1002/jmri.27596 EDN: SFBRHR
- Rowe SP, Pienta KJ, Pomper MG, Gorin MA. PSMA-RADS Version 1.0: a step towards standardizing the interpretation and reporting of PSMA–targeted PET imaging studies. European Urology. 2018;73(4):485–487. doi: 10.1016/j.eururo.2017.10.027
- Vasilev YA, Omelyanskaya OV, Vladzymyrskyy AV, et al. Comparison of multiparametric and biparametric magnetic resonance imaging protocols for prostate cancer diagnosis by radiologists with different experience. Digital Diagnostics. 2023;4(4):455–466. doi: 10.17816/dd322816 EDN: PVEPWX
- Disler DG, McCauley TR, Ratner LM, et al. In-phase and out-of-phase MR imaging of bone marrow: prediction of neoplasia based on the detection of coexistent fat and water. American Journal of Roentgenology. 1997;169(5):1439–1447. doi: 10.2214/ajr.169.5.9353477
- Suh CH, Yun SJ, Jin W, et al. Diagnostic Performance of in-phase and opposed-phase chemical-shift imaging for differentiating benign and malignant vertebral marrow lesions: a meta-analysis. American Journal of Roentgenology. 2018;211(4):W188–W197. doi: 10.2214/AJR.17.19306
- Halpern SD. The continuing unethical conduct of underpowered clinical trials. JAMA. 2002;288(3):358–362. doi: 10.1001/jama.288.3.358
- Altman DG. Statistics and ethics in medical research: III How large a sample? BMJ. 1980;281(6251):1336–1338. doi: 10.1136/bmj.281.6251.1336
- Blokhin IA, Kodenko MR, Shumskaya YuF, et al. Hypothesis testing using R. Digital Diagnostics. 2023;4(2):238–247. doi: 10.17816/DD121368 EDN: OEKDAG
- Grünig H, Maurer A, Thali Y, et al. Focal unspecific bone uptake on [18F]-PSMA-1007 PET: a multicenter retrospective evaluation of the distribution, frequency, and quantitative parameters of a potential pitfall in prostate cancer imaging. European Journal of Nuclear Medicine and Molecular Imaging. 2021;48(13):4483–4494. doi: 10.1007/s00259-021-05424-x
- Silver DA, Pellicer I, Fair WR, et al. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997:3(1):81–85.
- Plouznikoff N, Garcia C, Artigas C, et al. Heterogeneity of 68Ga-PSMA PET/CT uptake in fibrous dysplasia. Clinical Nuclear Medicine. 2019;44(10):e593–e594. doi: 10.1097/RLU.0000000000002609
- Gossili F, Lyngby CG, Løgager V, Zacho HD. Intense PSMA uptake in a vertebral hemangioma mimicking a solitary bone metastasis in the primary staging of prostate cancer via 68Ga-PSMA PET/CT. Diagnostics. 2023;13(10):1730. doi: 10.3390/diagnostics13101730 EDN: HQPGMR
- Hoyle JM, Layfield LJ, Crim J. The lipid-poor hemangioma: an investigation into the behavior of the “atypical” hemangioma. Skeletal Radiology. 2020;49:93–100. doi: 10.1007/s00256-019-03257-2
- Liao Z, Liu G, Ming B, et al. Evaluating prostate cancer bone metastasis using accelerated whole-body isotropic 3D T1-weighted Dixon MRI with compressed SENSE: a feasibility study. European Radiology. 2023;33(3):1719–1728. doi: 10.1007/s00330-022-09181-9
- Park S, Park JG, Jun S, et al. Differentiation of bone metastases from prostate cancer and benign red marrow depositions of the pelvic bone with multiparametric MRI. Magnetic Resonance Imaging. 2020;73:118–124. doi: 10.1016/j.mri.2020.08.019 EDN: CTHKSL
- Lee JH, Park S. Differentiation of schmorl nodes from bone metastases of the spine: use of apparent diffusion coefficient derived from DWI and fat fraction derived from a Dixon sequence. American Journal of Roentgenology. 2019;213(5):W228–W235. doi: 10.2214/AJR.18.21003
- Hottat NA, Badr DA, Ben Ghanem M, et al. Assessment of whole-body MRI including diffusion-weighted sequences in the initial staging of breast cancer patients at high risk of metastases in comparison with PET-CT: a prospective cohort study. European Radiology. 2023;34(1):165–178. doi: 10.1007/s00330-023-10060-0 EDN: MRFKMJ
- Johnston EW, Latifoltojar A, Sidhu HS, et al. Multiparametric whole-body 3.0-T MRI in newly diagnosed intermediate- and high-risk prostate cancer: diagnostic accuracy and interobserver agreement for nodal and metastatic staging. European Radiology. 2018;29(6):3159–3169. doi: 10.1007/s00330-018-5813-4 EDN: DEXLFX
- Liu F, Dong J, Shen Y, et al. Comparison of PET/CT and MRI in the diagnosis of bone metastasis in prostate cancer patients: a network analysis of diagnostic studies. Frontiers in Oncology. 2021;11(APR):736654. doi: 10.3389/fonc.2021.736654 EDN: TKTQOV
- Nakanishi K, Tanaka J, Nakaya Y, et al. Whole-body MRI: detecting bone metastases from prostate cancer. Japanese Journal of Radiology. 2021;40(3):229–244. doi: 10.1007/s11604-021-01205-6 EDN: QZBDSB
- Sun W, Li M, Gu Y, et al. Diagnostic value of whole-body DWI with background body suppression plus calculation of apparent diffusion coefficient at 3 T Versus 18F-FDG PET/CT for detection of bone metastases. American Journal of Roentgenology. 2020;214(2):446–454. doi: 10.2214/ajr.19.21656 EDN: BJRCLP
- Larbi A, Omoumi P, Pasoglou V, et al. Whole-body MRI to assess bone involvement in prostate cancer and multiple myeloma: comparison of the diagnostic accuracies of the T1, short tau inversion recovery (STIR), and high b-values diffusion-weighted imaging (DWI) sequences. European Radiology. 2018;29(8):4503–4513. doi: 10.1007/s00330-018-5796-1 EDN: CEOKNS
- Chen B, Wei P, Macapinlac HA, Lu Y. Comparison of 18F-Fluciclovine PET/CT and 99mTc-MDP bone scan in detection of bone metastasis in prostate cancer. Nuclear Medicine Communications. 2019;40(9):940–946. doi: 10.1097/MNM.0000000000001051 EDN: ZRPGWP
- Gelezhe P.B. Comprehensive diagnostics of breast cancer using magnetic resonance imaging and positron emission tomography with 18F-fluorodeoxyglucose, combined with computed tomography [dissertation]. Moscow; 2020. Available from: https://www.elibrary.ru/item.asp?id=54413422 EDN: UGEBZO
- Freitag MT, Radtke JP, Hadaschik BA, et al. Comparison of hybrid 68Ga-PSMA PET/MRI and 68Ga-PSMA PET/CT in the evaluation of lymph node and bone metastases of prostate cancer. European Journal of Nuclear Medicine and Molecular Imaging. 2015;43(1):70–83. doi: 10.1007/s00259-015-3206-3 EDN: HXYHGT
Supplementary files